
|
Published: 10 November 2014
Polar underwater lab to study seafloor acidification
Scientists will simulate a future ocean floor under the sea ice off the Australian Antarctic station at Casey this summer to observe the potential impact of ocean acidification on seafloor communities.
|
|
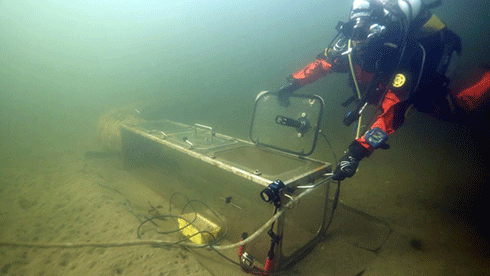
A diver tests one of the chambers in the cold waters of Tasmania before deployment in Antarctica Credit:
Jonny Stark
|
Ocean acidification is caused by increasing amounts of atmospheric carbon dioxide (CO2) dissolving into seawater. Dissolved CO2 causes the pH of the seawater to drop and become more acidic. This affects the ability of some marine organisms, including corals and bivalves, to form shells and other hard structures.
The Southern Ocean absorbs 40 per cent of the global ocean uptake of CO2 as cold water is able to absorb more CO2 than warmer water. As a result, polar waters are acidifying at twice the rate of tropical waters.
Over the past 300 million years ocean acidity, measured by its pH, has been slightly basic, and averaged about 8.2 (a pH of 7 is neutral). Current atmospheric CO2 concentrations are about 416 parts per million (ppm), while ocean pH has dropped to 8.1 since pre-industrial times – a 25 per cent increase in acidity over two centuries. By 2100, under business as usual emissions, atmospheric CO2 is predicted to be about 936 ppm and ocean pH 7.8.
To test the effects of this increasing acidity, four semi-enclosed chambers are being deployed 10 to 20 m beneath the Antarctic sea ice off Casey between November 2014 and March 2015 as part of the Antarctic Free Ocean Carbon Enrichment experiment.
A team of scientists, divers, technicians and engineers will increase CO2 concentrations in the water within two of the chambers. This will decrease the pH of the water by 0.4 pH units, without changing light or nutrient concentrations.
A further two chambers will be used as controls to track natural fluctuations in pH in the surrounding water. This will allow the team to compare the response of benthic (ocean floor) communities exposed to current seawater pH levels, and the more acidic pH levels predicted under future CO2 emission scenarios.
Researchers will measure parameters such as:
-
changes in benthic invertebrate community biodiversity and composition
-
seawater carbonate chemistry
-
nutrient cycling and animal-driven turbidity in sediment
-
changes in communities of bacteria, diatoms (single celled marine plants) and sediment meiofauna (very tiny marine animals)
Ocean acidification disrupts the formation of calcium carbonate (CaCO3), which is a major structural component of shells and similar hard structures made by some marine organisms, including phytoplankton and coral. It also affects the metabolic and physiological processes inside organisms including development, growth, reproduction and respiration.
The Casey research will assist governments, scientists, modellers and society to understand the emerging impacts of ocean acidification on marine ecosystems and to ensure that the most relevant information underpins decisions to manage the threat.
Source: Australian Antarctic Division



